
PIPELINE Precision Cardiology
Delivering on the RNA Revolution
We are advancing and expanding our innovative AOC pipeline to offer treatment options for patients and their families across a wide range of therapeutic areas. We have three AOC programs for three distinct rare diseases in clinical development from our muscle disease franchise: myotonic dystrophy type 1 (DM1), facioscapulohumeral muscular dystrophy (FSHD), and Duchenne Muscular Dystrophy (DMD). Our pipeline also includes advancing AOCs to address additional DMD, rare neuromuscular, and rare precision cardiology programs. We continue to broaden our reach of AOCs in other indications including cardiology and immunology through partnerships.
Precision Cardiology
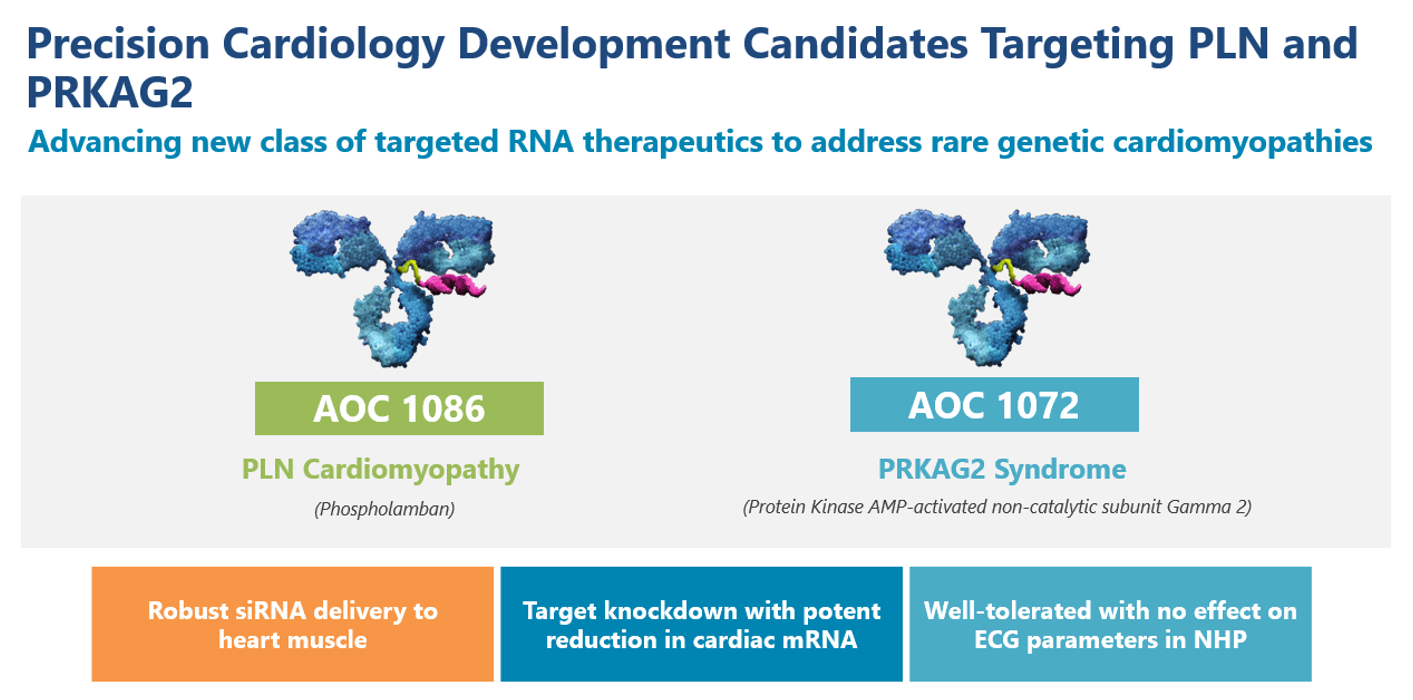
Avidity has expanded beyond rare skeletal muscle disorders and is opening up a new therapeutic field, precision cardiology, to address the root cause of genetic diseases of the heart. Avidity is advancing its first two wholly-owned precision cardiology development candidates targeting rare genetic cardiomyopathies: AOC 1086 targeting PLN (phospholamban) cardiomyopathy and AOC 1072 targeting PRKAG2 (Protein Kinase AMP-activated non-catalytic subunit Gamma 2) Syndrome.
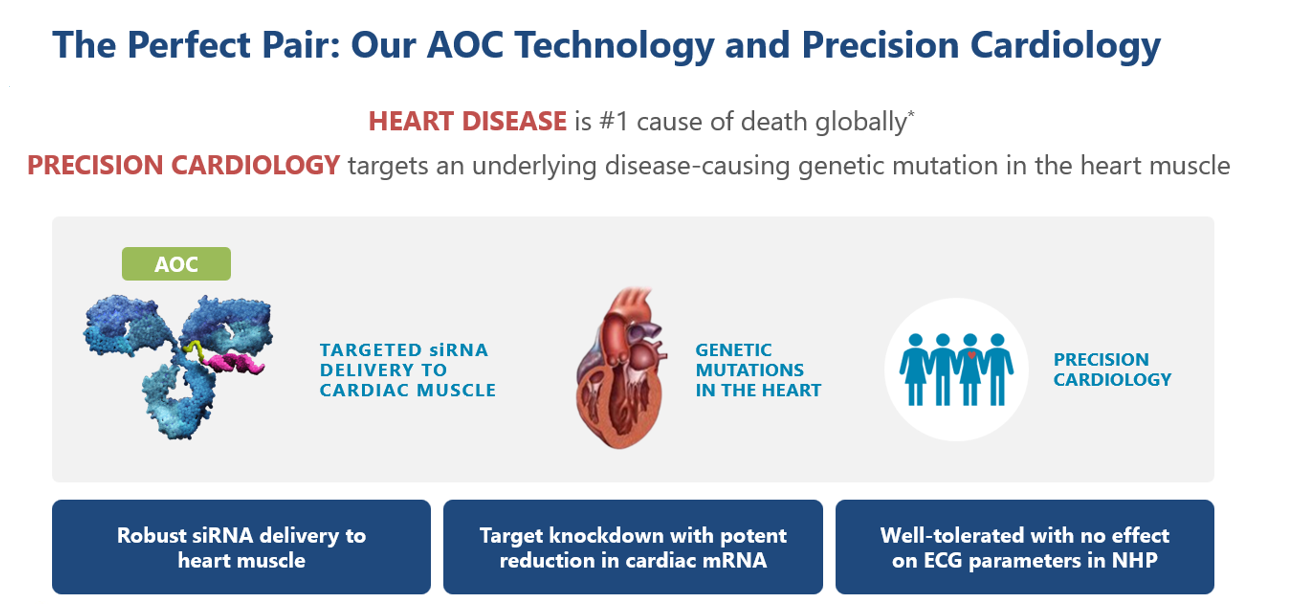
AOC 1086 and AOC 1072 are designed to deliver siRNA directly to the heart muscle to reduce the expression of PLN and PRKAG2 genes, respectively. Pathogenic mutations in those genes cause rare genetic cardiomyopathies. In preclinical studies, AOC 1086 and AOC 1072 demonstrated robust siRNA delivery to the heart muscle and potent targeted knockdown of approximately 80% in cardiac PLN mRNA and PRKAG2 mRNA. AOC 1086 and AOC 1072 were well tolerated without any effect on electrocardiogram parameters for evaluating cardiac safety in preclinical studies.
PRKAG2 Preclinical Data at American Heart Association (AHA) Scientific Sessions 2024
Preclinical data from AOC 1072 targeting PRKAG2 was presented at the American Heart Association (AHA) Scientific Sessions 2024 on November 16, 2024 in Chicago, Illinois.
PLN Cardiomyopathy
PLN (Phospholamban) cardiomyopathy is an autosomal dominant, progressive cardiac disease caused by a mutation in the PLN gene. The most common disease variant is the R14del, causing a buildup of toxic protein aggregates that trigger cardiomyocyte death leading to dilation of the heart chambers, arrhythmias (irregular heartbeats), sudden cardiac arrest and heart failure. There are no approved disease-modifying therapies for PLN cardiomyopathy, and current standard of care focuses on symptom management with medications or invasive treatments including pacemakers or implantable cardioverter defibrillator (ICD). Patients who develop progressive heart failure may require a heart transplant.
PRKAG2 Syndrome
PRKAG2 (Protein Kinase AMP-activated non-catalytic subunit Gamma 2) Syndrome is an autosomal dominant, progressive cardiac disease caused by a mutation in the PRKAG2 gene that results in increased AMP-activated protein kinase (AMPK) activity. This causes excessive cardiac glycogen storage in the heart, leading to cardiac hypertrophy (thickening of heart muscle) and multiple different types of arrhythmias (irregular heartbeats), including Wolff-Parkinson-White syndrome, atrial fibrillation, and cardiac conduction system disease. As a result, patients with PRKAG2 syndrome are at an increased risk of experiencing sudden cardiac arrest or heart failure. There are no approved disease-modifying therapies for PRKAG2 Syndrome, and current standard of care focuses on symptom management with medications or invasive treatments including pacemakers or implantable cardioverter defibrillator (ICD). Patients who develop progressive heart failure may require a heart transplant.