The evolution of RNA technology
ANTIBODY OLIGONUCLEOTIDE CONJUGATES (AOCs) DEFINED
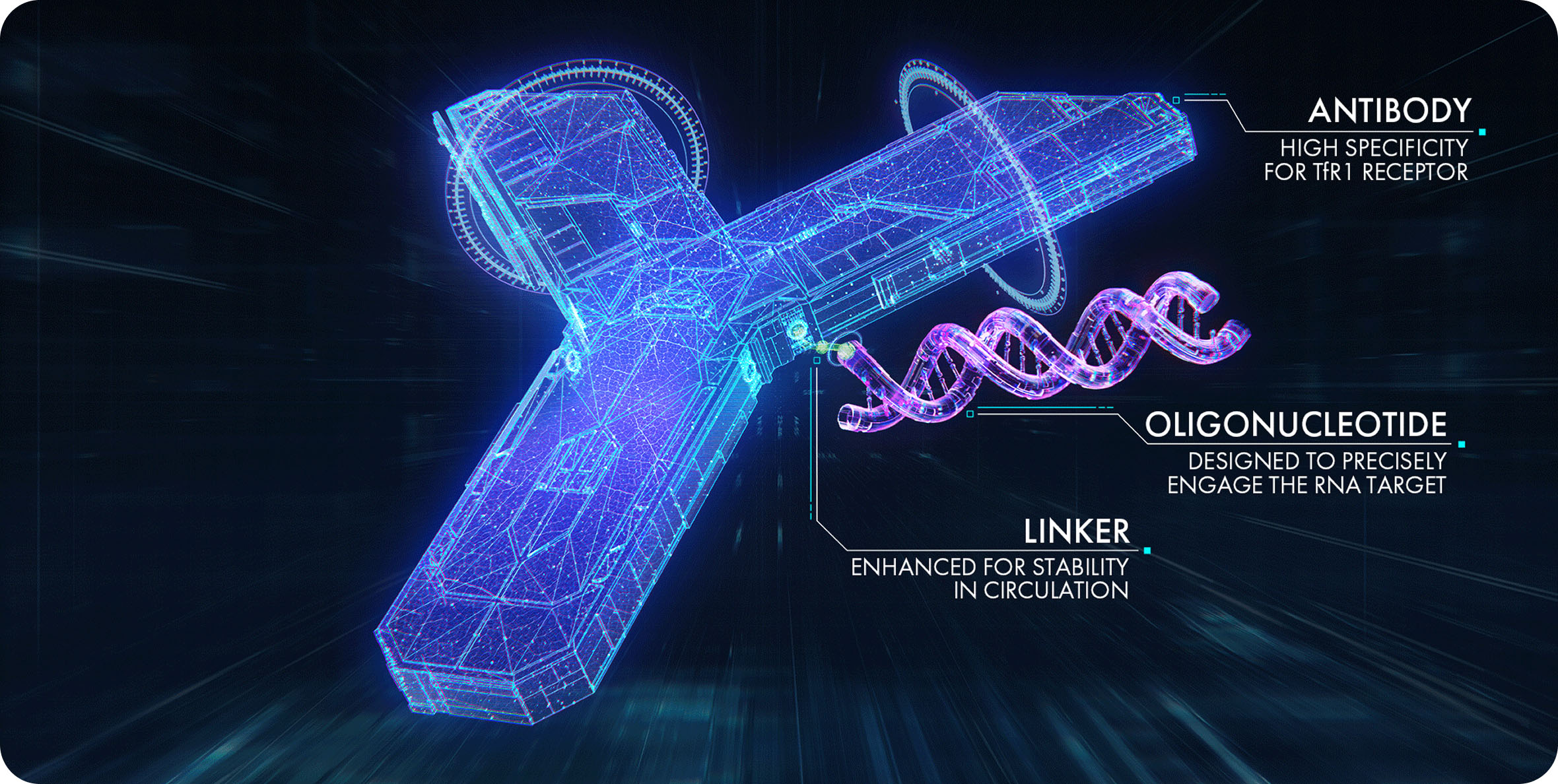
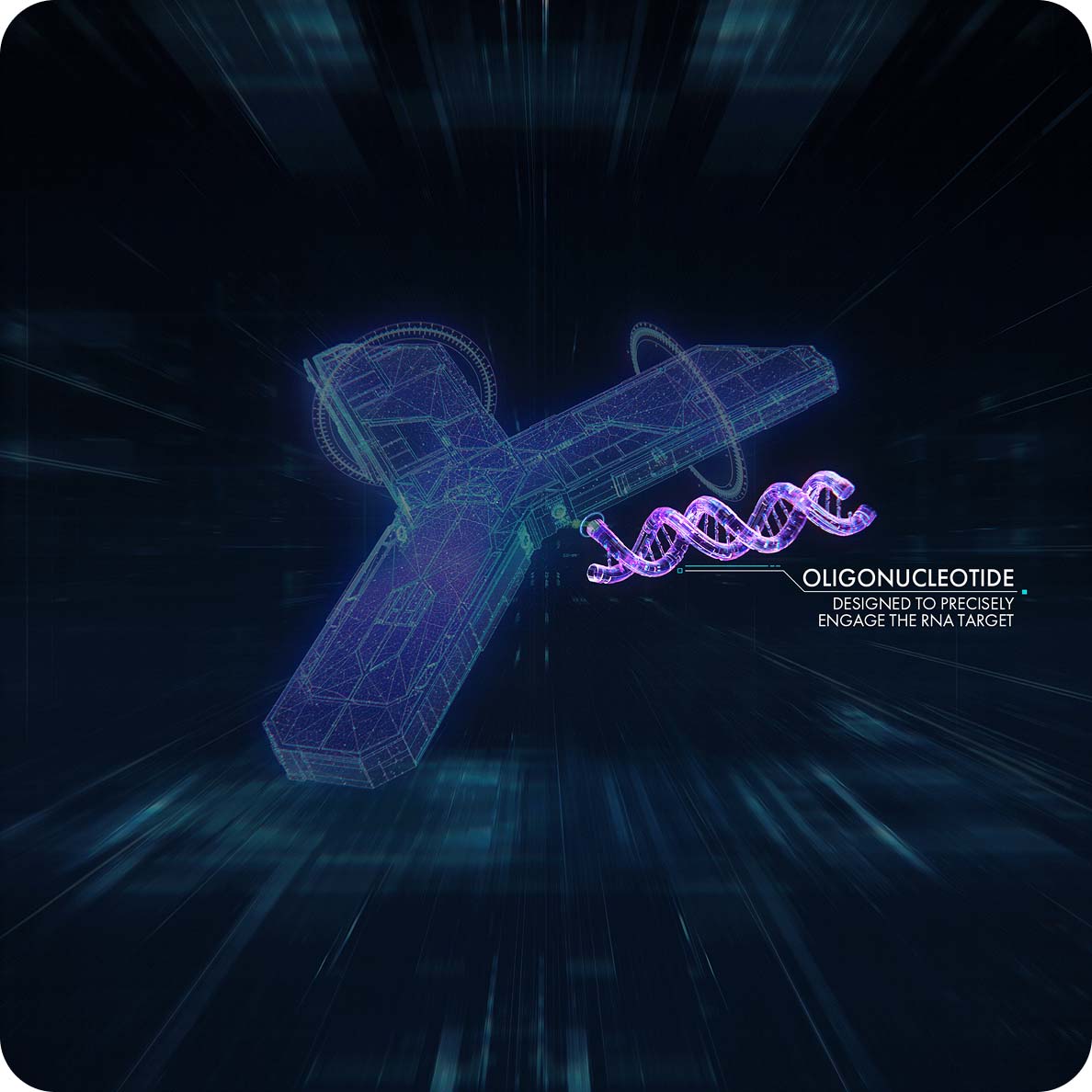
AOCs are 3-part molecules consisting of a monoclonal antibody that is conjugated via a linker to one or more oligonucleotides (a small-interfering RNA [siRNA] or phosphonodiamidite morpholino oligomer [PMO]), with the aim of delivering RNA therapy to muscle.1,2
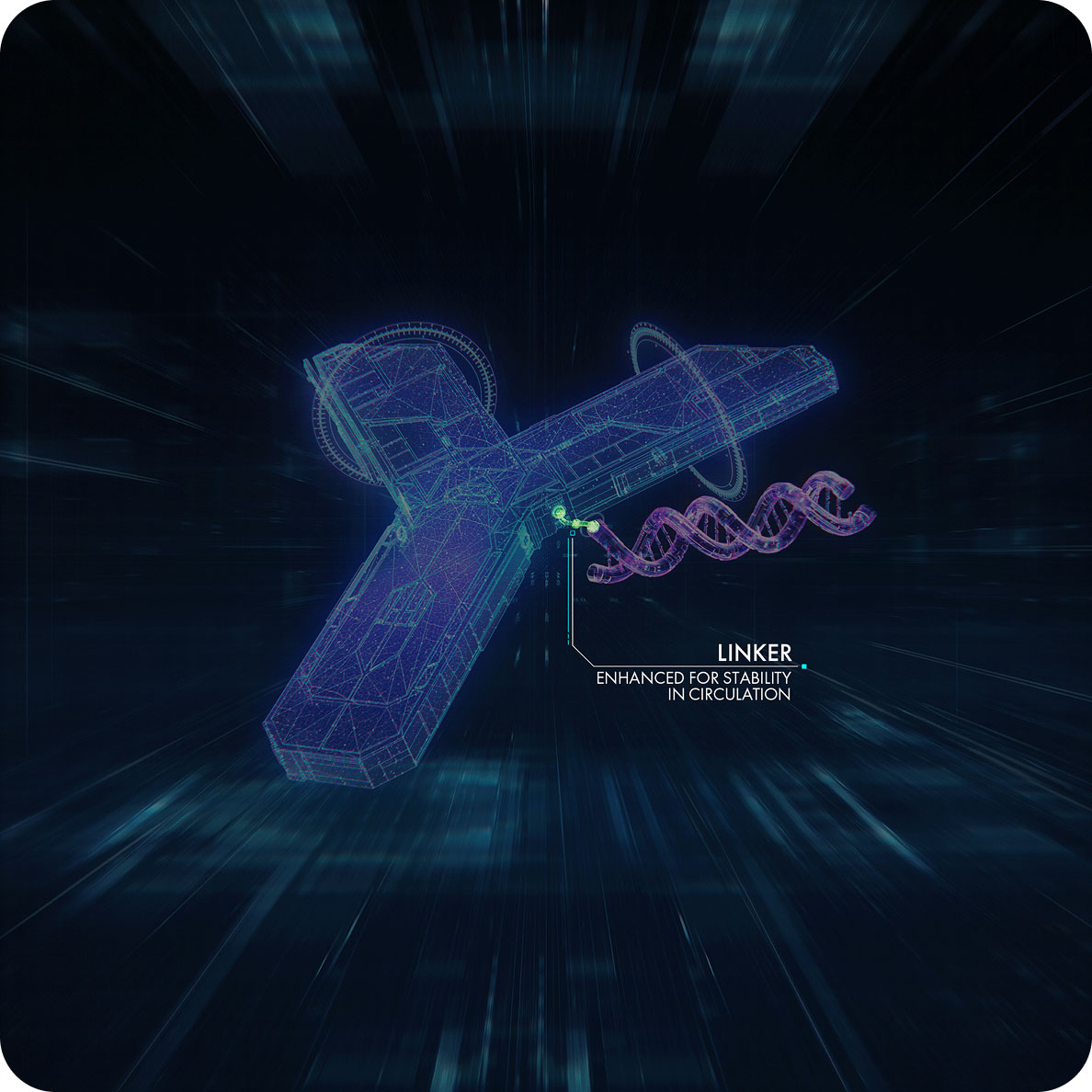
Depending on the underlying pathophysiological mechanism, siRNA or PMO is selected to be conjugated to the monoclonal antibody, providing a means by which the oligonucleotide can be efficiently delivered to the RNA target.2
The monoclonal antibody portion of AOCs is designed to target the TfR1 receptor, which is highly expressed by muscle cells. After the siRNA or PMO separates from the antibody, the oligonucleotides can bind to their RNA targets and modulate gene expression.2,3
The monoclonal antibodies have been specifically designed to target the TfR1 receptor with high specificity and affinity, enabling precise delivery of therapies to targeted tissues such as muscle cells.2
Speak with a medical science liaison
ConnectPotential of aocs
Rapid advances in the last few decades have led to multiple approvals of oligonucleotide therapeutics across indications.5
Effective systemic delivery of oligonucleotide therapeutics requires innovative engineering at multiple levels to potentially achieve safe and effective delivery to target tissues.6
Avidity Biosciences was the first company to demonstrate the successful targeted delivery of RNA to muscle with systemic administration in clinical trials2,7,8
The proprietary AOC platform was designed to2,7,8:
- Combine the specificity of monoclonal antibodies (mAbs) with precise and potent oligonucleotides in one therapeutic conjugate
- Enable targeted delivery to affected tissues and address the root cause of disease
Stay up to date on the latest innovations
sign-up for updatesAvidity’s proprietary aoc platform
PRECISION-ENGINEERED AOCs UNLOCK TARGETED RNA DELIVERY TO TISSUES2
Avidity's AOCs are purposefully engineered to reach previously unreachable cell types with potentially fewer off-target effects.1,2
Each component of the AOC platform is engineered with the intent to increase precision, durability, potency, and safety—with the ultimate goal of providing customized treatments for a range of genetic neuromuscular disorders.1,2
Advancing Research & Development of the AOC Platform
Avidity is now expanding research and development of RNA therapeutics beyond neuromuscular diseases into new therapeutic areas, such as cardiology and immunology.
Avidity is committed to redefining what’s possible for people living with rare neuromuscular diseases—such as Duchenne muscular dystrophy (DMD), facioscapulohumeral muscular dystrophy (FSHD), and myotonic dystrophy type 1 (DM1)—through investigation and development of RNA-targeted therapeutics powered by its proprietary AOC platform.
AOC=antibody oligonucleotide conjugate; ED50=effective dose in 50% of the population; mAb=monoclonal antibody; PMO=phosphorodiamidate morpholino oligomer; siRNA=short interfering RNA; DMD=Duchenne muscular dystrophy; FSHD=facioscapulohumeral muscular dystrophy; DM1= myotonic dystrophy type 1.
References:
- Cochran M, Arias D, Burke R, et al. Structure-Activity Relationship of Antibody-Oligonucleotide Conjugates: Evaluating Bioconjugation Strategies for Antibody-siRNA Conjugates for Drug Development. J Med Chem. 2024;67(17):14852-14867. doi:10.1021/acs.jmedchem.4c00802
- Malecova B, Burke RS, Cochran M, et al. Targeted tissue delivery of RNA therapeutics using antibody-oligonucleotide conjugates (AOCs). Nucleic Acids Res. 2023;51(12):5901-5910. doi:10.1093/nar/gkad415
- Li Y, Cheng JX, Yang HH, et al. Transferrin receptor 1 plays an important role in muscle development and denervation-induced muscular atrophy. Neural Regen Res. 2021;16(7):1308-1316. doi:10.4103/1673-5374.301024
- Moumné L, Marie AC, Crouvezier N. Oligonucleotide Therapeutics: From Discovery and Development to Patentability. Pharmaceutics. 2022;14(2):260. Published 2022 Jan 22. doi:10.3390/pharmaceutics14020260
- Lauffer MC, van Roon-Mom W, Aartsma-Rus A; N = 1 Collaborative. Possibilities and limitations of antisense oligonucleotide therapies for the treatment of monogenic disorders. Commun Med (Lond). 2024;4(1):6. Published 2024 Jan 5. doi:10.1038/s43856-023-00419-1
- Roberts TC, Langer R, Wood MJA. Advances in oligonucleotide drug delivery. Nat Rev Drug Discov. 2020;19(10):673-694. doi:10.1038/s41573-020-0075-7
- PR Newswire 2022
- Johnson N, Day J, Hamel J, et al. Topline data analysis of the Phase 1/2 clinical trial evaluating AOC 1001 in adult patients with myotonic dystrophy type 1: MARINA™. Poster presented at: 28th Annual Congress of the World Muscle Society; October 3–7, 2023; Charleston, SC.
© 2025 Avidity Biosciences. The Avidity logo is a registered trademark of Avidity Biosciences. All rights reserved.
MAT-US-AOC-250010 v2.0 07/25