
PIPELINE FSHD
Delivering on the RNA Revolution
We are advancing and expanding our innovative AOC pipeline to offer treatment options for patients and their families across a wide range of therapeutic areas. We have three AOC programs for three distinct rare diseases in clinical development from our muscle disease franchise: myotonic dystrophy type 1 (DM1), facioscapulohumeral muscular dystrophy (FSHD), and Duchenne Muscular Dystrophy (DMD). Our pipeline also includes advancing AOCs to address additional DMD, rare neuromuscular, and rare precision cardiology programs. We continue to broaden our reach of AOCs in other indications including cardiology and immunology through partnerships.
Facioscapulohumeral Muscular Dystrophy
Facioscapulohumeral muscular dystrophy (FSHD) is a rare, progressive, and variable hereditary muscle-weakening condition marked by life-long, relentless loss of muscle function, significant pain, fatigue, and progressive disability. It is one of the most common forms of muscular dystrophy, with onset typically in teens and young adults. We estimate that the FSHD patient population is between 45,000-87,000 in the United States and Europe. Currently, there are no approved treatments for people living with FSHD. Current treatment approaches are focused on support for activities of daily living and mobility, improved functioning and lowering the risk of complications. They include physical therapy, exercise, pain management and orthopedic interventions.
FSHD is characterized by progressive and often asymmetric skeletal muscle loss that initially causes weakness in muscles in the face, shoulders, arms and trunk and progresses to weakness in muscles in the lower body. FSHD is an autosomal dominant genetic disease caused by the aberrant expression of the DUX4 (double homeobox 4) gene in the skeletal muscle, which activates genes that are toxic to muscle cells and leads to a series of downstream events that result in skeletal muscle wasting and compromised muscle function. Skeletal muscle weakness results in physical limitations throughout the whole body including an inability to lift arms for more than a few seconds, loss of ability to show facial expressions, and serious speech impediments. These symptoms cause many people affected by FSHD to become dependent on the use of a wheelchair for mobility.
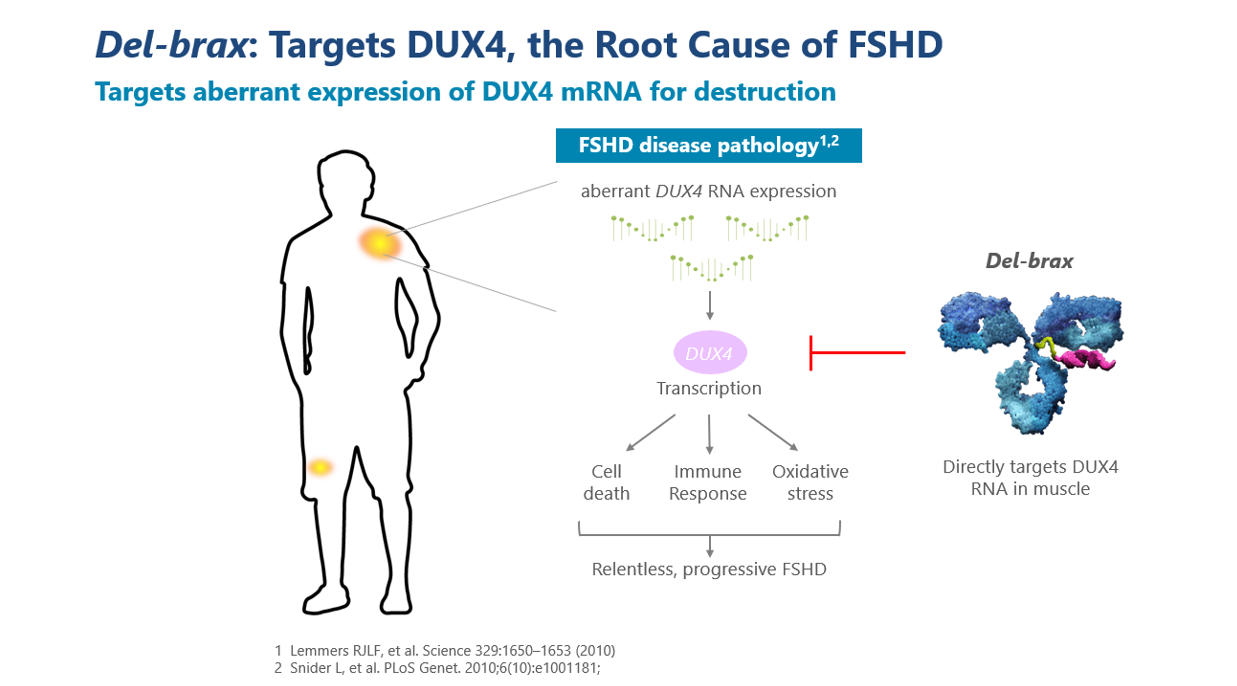
Delpacibart braxlosiran or del-brax
Del-brax is designed to treat the underlying cause of FSHD, which is caused by the abnormal expression of a gene called double homeobox 4 or DUX4. The abnormal expression of DUX4 protein leads to changes in gene expression in muscle cells that are associated with the life-long, progressive loss of muscle function in patients with FSHD. Del-brax aims to reduce the expression of DUX4 mRNA and DUX4 protein in muscles in people with FSHD. Del-brax consists of a proprietary monoclonal antibody that binds to the transferrin receptor 1 (TfR1) conjugated with a siRNA targeting DUX4 mRNA. Avidity reported positive initial del-brax 2 mg/kg data at four months from the Phase 1/2 FORTITUDE™ trial demonstrating unprecedented and consistent reductions of greater than 50% in DUX4 regulated genes, trends of functional improvement, and favorable safety and tolerability in people living with FSHD. Del-brax is currently in Phase 1/2 development as part of the FORTITUDE trial in individuals with FSHD. The U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA) have granted Orphan designation for del-brax and the FDA has granted del-brax Fast Track designation.
Biomarker Cohort Enrollment Completion in Phase 1/2 FORTITUDE™ Trial for Del-brax Announced in March 2025
In March 2025, we announced the completion of enrollment in the biomarker cohort for the Phase 1/2 FORTITUDE™ trial of del-brax. The ongoing biomarker cohort is designed for a potential accelerated approval path in the U.S. and plan to share a regulatory update on the accelerated approval path in the second quarter of 2025. The biomarker cohort is assessing the impact of del-brax 2 mg/kg every six weeks versus placebo for 12 months in people living with FSHD, ages 16-70. The primary endpoints of the study cohort are changes in DUX4-regulated gene expression and DUX4-regulated circulating biomarker.
Preliminary Data Presented at 2024 FSHD Society International Research Congress
In June 2024, Avidity announced unprecedented data from the Phase 1/2 FORTITUDE™ trial in people living with FSHD. Del-brax data demonstrated unprecedented and consistent reductions of greater than 50% in DUX4 regulated genes, trends of functional improvement, and favorable safety and tolerability in people living with facioscapulohumeral muscular dystrophy (FSHD). Trends of functional improvements including increased strength in upper and lower limb muscles, and muscle function as measured by reachable workspace (RWS) compared to placebo and the ReSolve natural history study were observed. Data on DUX4 regulated genes, circulating biomarkers and muscle strength and function were assessed from 12 participants in the 2 mg/kg cohort.

The FORTITUDE™ trial is a randomized, placebo-controlled, double-blind, Phase 1/2 clinical trial designed to evaluate single and multiple doses of delpacibart braxlosiran or del-brax in 90 participants with facioscapulohumeral muscular dystrophy (FSHD). FORTITUDE is evaluating the safety, tolerability, pharmacokinetics, and pharmacodynamics of del-brax administered intravenously. Activity of del-brax is being assessed using key biomarkers, including DUX4-regulated muscle and circulating biomarkers and magnetic resonance imaging (MRI) measures of muscle volume and composition. Though the Phase 1/2 trial is not statistically powered to assess functional benefit, it explores the clinical activity of del-brax including measures of mobility and muscle strength as well as patient reported outcomes and quality of life measures.
The trial has a total of three dose cohorts. The first two dose escalation cohorts evaluated 2 mg/kg or 4 mg/kg of del-brax versus placebo and were designed to assess safety as well as inform the dose and dose regimen of del-brax for additional studies. Avidity has completed enrollment in the dose escalation cohorts and identified 2 mg/kg every six weeks of del-brax as the dose for future clinical trials.
The third, ongoing biomarker cohort in the FORTITUDE trial is designed for a potential accelerated approval path in the U.S. It is assessing the impact of del-brax 2 mg/kg every six weeks versus placebo for 12 months in people living with FSHD, ages 16-70. The primary endpoints of the study cohort are changes in DUX4-regulated gene expression and DUX4-regulated circulating biomarker. Enrollment in the biomarker cohort is complete with a total number of 51 participants enrolled.
Participants who complete FORTITUDE have the option to enroll in the ongoing FORTITUDE open-label extension (FORTITUDE-OLE™) study evaluating the long-term safety and tolerability of del-brax in participants living with FSHD. For more information about the FORTITUDE trial, visit the FORTITUDE study website or visit http://www.clinicaltrials.gov and search for NCT05747924.

FORTITUDE-OLE™ is an open-label, multi-center trial designed to evaluate the long-term safety and tolerability of delpacibart braxlosiran or del-brax in participants with facioscapulohumeral muscular dystrophy (FSHD) who were previously enrolled in the Phase 1/2 FORTITUDE™ trial. This trial will continue to evaluate the safety, tolerability, PK, PD, and efficacy of del-brax in participants who enrolled in the randomized, placebo-controlled, Phase 1/2 FORTITUDE clinical trial. Participants who enroll in the FORTITUDE-OLE study will receive del-brax regardless of whether they received active treatment or placebo in the FORTITUDE study. The total duration of active treatment with del-brax in the FORTITUDE-OLE is approximately 24 months. Avidity may extend active treatment beyond 24 months at a future timepoint. For more information on the FORTITUDE-OLE study click here or visit https://www.clinicaltrials.gov and search for NCT06547216.