Pipeline Overview
Delivering on the RNA Revolution
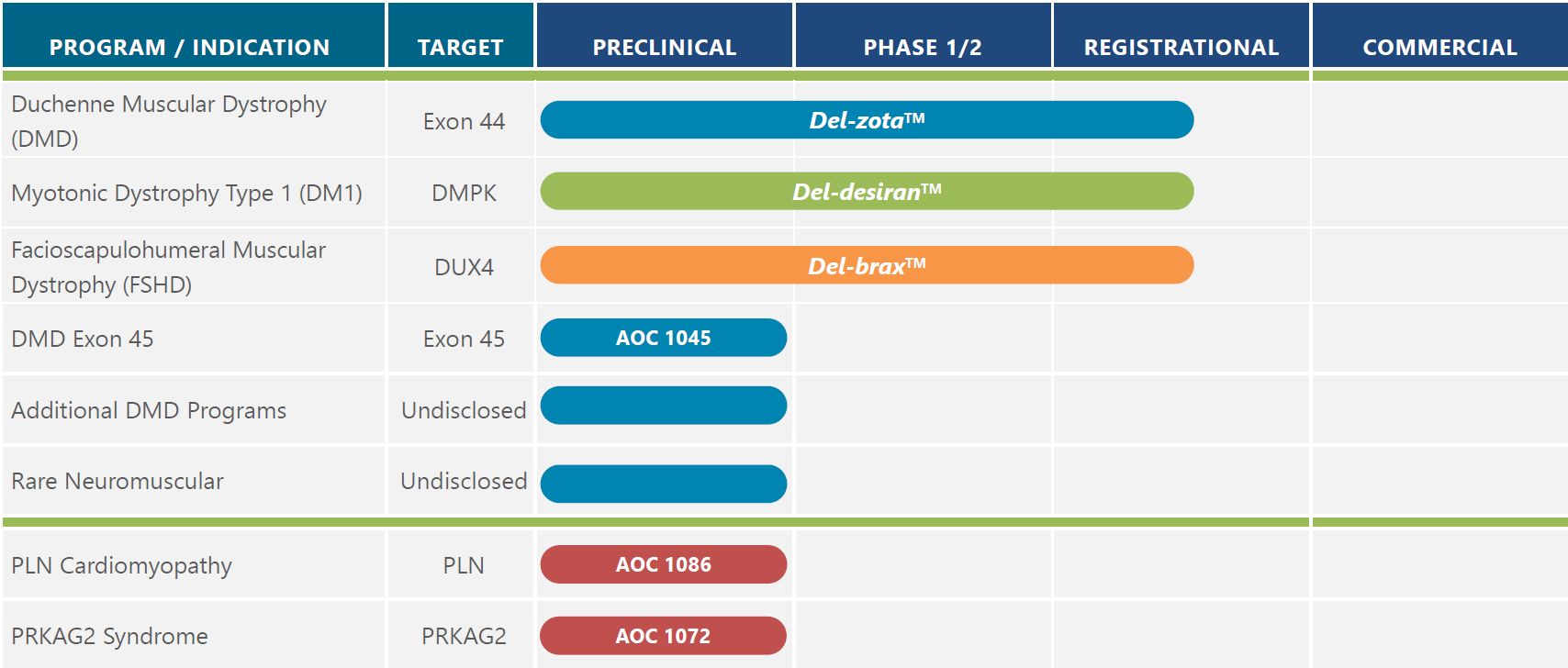
We are advancing and expanding our innovative AOC pipeline to offer treatment options for patients and their families across a wide range of therapeutic areas. We have three AOC programs for three distinct rare diseases in clinical development from our muscle disease franchise: myotonic dystrophy type 1 (DM1), facioscapulohumeral muscular dystrophy (FSHD), and Duchenne Muscular Dystrophy (DMD). Our pipeline also includes advancing AOCs to address additional DMD, rare neuromuscular, and rare precision cardiology programs. We continue to broaden our reach of AOCs in other indications including cardiology and immunology through partnerships.